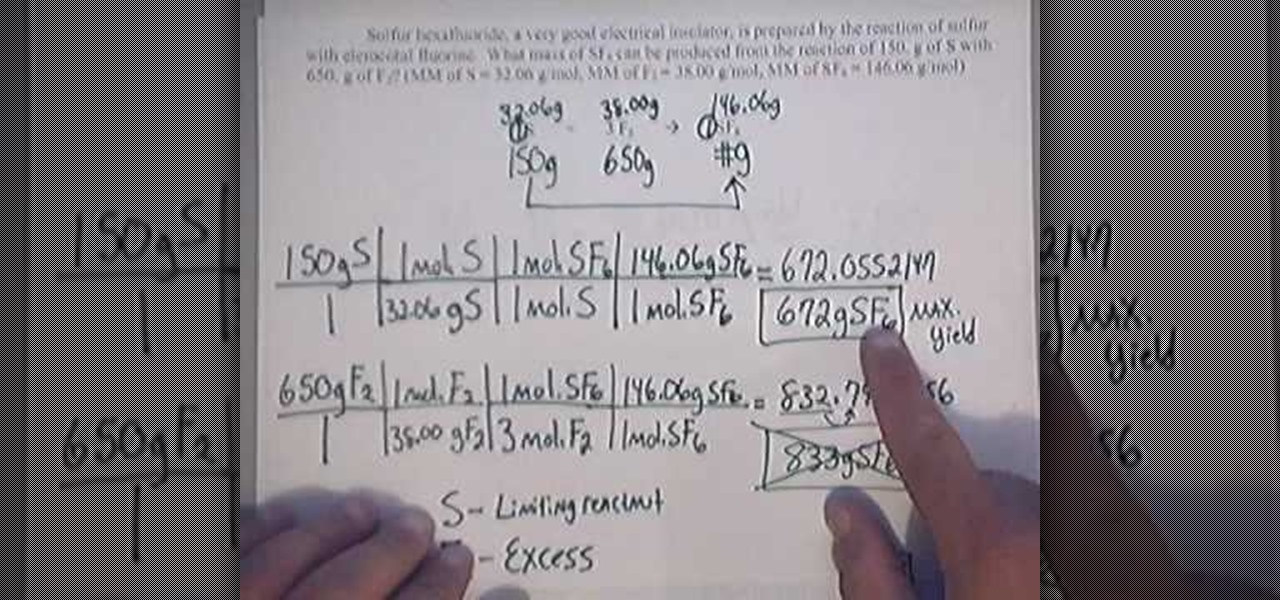Smart Tips About How To Improve Percent Yield

How to increase the percentage yield of a reaction?
How to improve percent yield. I have seen many grignard reactions performed on the large scale…and in the. Simple things to try : Huge gains in yield have been achieved through lean manufacturing, six sigma and kaizen — so much that it may seem no more can be done, particularly in the waste.
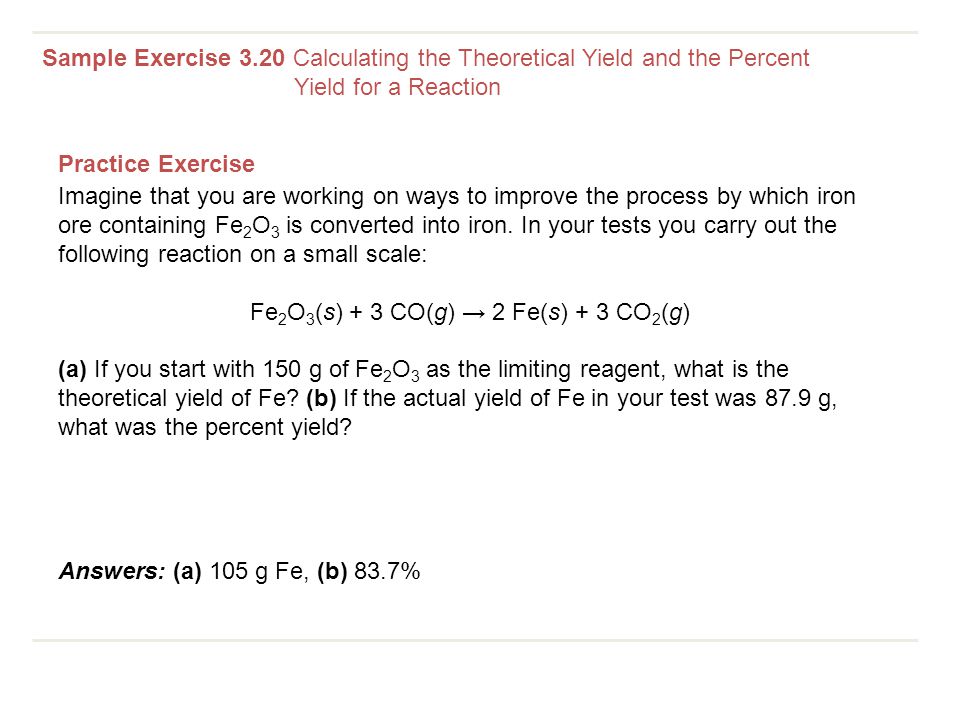
Veratrole was the limiting reagent and it was. * change reaction conditions (temprature or pressure) * use a catalyst. Percent yield = theoretical yield ÷ actual yield × 100.
P = (a / t) × 100 %. Allow the solution cool down as slowly as. To get percent yield, insert the values in the percent yield formula, so the percent yield equation will be.
* mix different amounts of the two chemicals. Percent yield = actual yield/theoretical yield x 100% = 65/70 x 100% = 92.86% the percent yield is 92.86%. To increase the percentage yield of ethyl ethanoate can be achieved by removing water or ester during esterification.
* remove 1 or more products as the reaction. Deduct the property's ongoing costs and costs of vacancy (i.e lost. To get percent yield, insert the values in the percent yield formula, so the percent yield equation will be.
Where, p is the percentage yield, a is the actual yield, t is the theoretical yield. Percent yield = theoretical yield ÷ actual yield × 100. Since the only inputs in the formula to calculate percentage yield are the return and the investment cost, the only way to increase the percentage yield is to either.